|
 |
| |
| ¡@ |
| Redox in Pathogen-Host Cell Interplay |
Certain bacteria synthesize glutathionyl spermidine (Gsp), from glutathione (GsH) and spermidine. E. coli Gsp synthetase/amidase (GspSA) catalyzes both the synthesis and hydrolysis of Gsp.Prior to our results,the physiological role of Gsp and how the two opposing GspSA activities are regulated had not been elucidated.
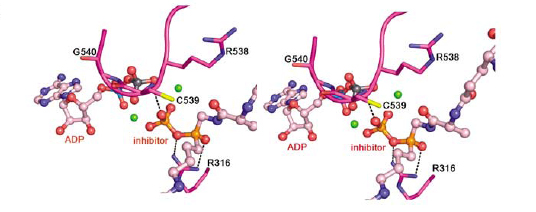
We are the first to discover that Gsp-modified proteins from E. coli contain mixed disulfides of Gsp and protein thiols, representing a new type of post-translational modification formally undocumented. The level of these proteins is increased by oxidative stress. We attribute the accumulation of such proteins to the selective inactivation of Gsp amidase activity. X-ray crystallography and a chemical modification study indicated that the catalytic cysteine thiol of the Gsp amidase domain is transiently inactivated by H2O2 oxidation to sulfenic acid that is stabilized by a very short hydrogen bond with a water molecule. We propse a set of reactions that explain how the levels of Gsp and Gsp S-thiolated proteins are modulated in response to oxidative stress. The hypersesitivities of GspSA and GspSA/glutaedoxin null mutants to H2O2 support the idea that GspSA and glutaredoxin act synergistically to regulate the redox environment of E. coli.
The gene of GspSA has been found in a number of pathogenic bacteria, including Salmonella enterica, Klesiella pneumoniae and Shigella flexneri. Since these intestinal bacteria likely evolve special strategies for survival under oxidative stress, it will be intriguing if Gsp can S-thiolate proteins in these pathogens, and if their GspSAs are selectively inactivated by H2O2. Such investigations will help to determine if GspSA is linked to pathogenic defense mechanism and thus becomes a target for therapeutic intervention. Therefore Gsp and the corresponding protein modification may represent a special evolved strategy for survival. GspSA is likely a target for therapeutic intervention.
|
 |
 |
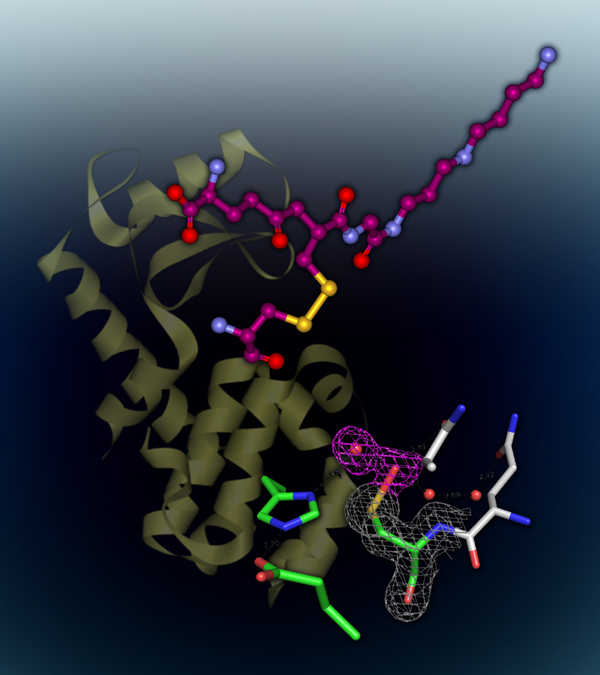 |
Protein Gsp S-thiolation and cysteine-sulfenic acid in Gsp amidase |
|
¡@ |
|
|
Browser & IE Recommendations: 1024*768 / IE 7.0¡BFirefox 3.0
Add : 128 Sec. 2, Academia Road, Nankang,Taipei 115,Taiwan¡@Tel : 886-2-27855696¡@Fax : 886-2-27889759 |
|
Copyright © 2010 IBC. All rights reserved. |
|
 |
![]() |