|
 |
| |
| ¡@ |
| Glycobiology in Pathogen-Host Cell Interplay |
Infecting about one-half of the global human population, Helicobacte. pylori is well established as the primary cause of gastritis, duodenal ulcer and gastric cancer. Currently there is no clear information regarding if and how host cells interact with H. pylori, and if such interactions are dependent on the type of gastric dieases. By using fluorescently labeled fucose-containing glycoconjugates, we provide the first evidence observing both the uptake of L-fucose from gastric cancer cells, and that human £\-L-fucosidase 2 (FUCA2) is secreted only under co-culture conditions. Upon depletion of FUCA2 by RNA interference and detection of translocated CagA (a virulence factor of H. pylori) in host cells, FUCA2 was found to be essential for H. pylori adhesion, in particular to the gastric cancer-and duodenal ulcer-specific strains. Additionally, FUCA2 was shown to significantly enhance the expression of Lewis x antigen in H. pylori, which is critical for bacteria cell adhesion in the pathogenesis and defense strategy to escape host surveillance. These findings not only demonstrate an important connection between FUCA2 and the adhesion, growth, and pathogenicity of H. pylori, but also support the idea FUCA2 is a potential target for clinical diagnosis and therapeutic intervention of H. pylori¡Vrelated diseases.
|
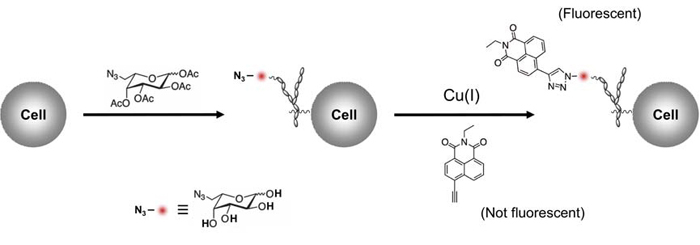 |
| Specific fluorescent labeling of fucosylated glycans in cells |
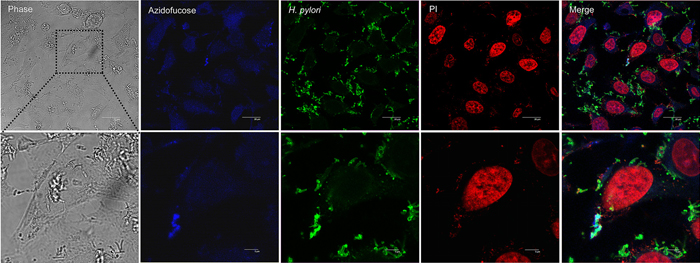 |
| Fucose transfer from gastric epithelial cells to H. pylori |
| |
Anti-H. pylori (Alexa Fluor 488, Green)
Nuclei-specific dye (DAPI, Red)
fucosylated glycans in host cells (Blue)
|
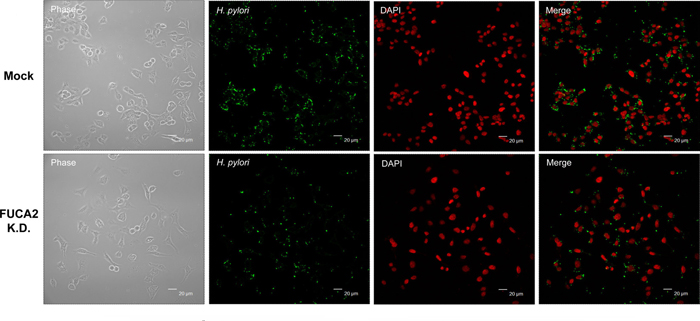 |
| FUCA2 is essential for the H. pylori adhesion to host cells |
| |
Anti-H. pylori (Alexa Fluor 488, Green)
Nuclei-specific dye (DAPI, Red)
|
|
¡@ |
|
|
Browser & IE Recommendations: 1024*768 / IE 7.0¡BFirefox 3.0
Add : 128 Sec. 2, Academia Road, Nankang,Taipei 115,Taiwan¡@Tel : 886-2-27855696¡@Fax : 886-2-27889759 |
|
Copyright © 2010 IBC. All rights reserved. |
|
 |
![]() |