|
 |
| |
| °@ |
| Glyco-enzymes in Drug Discovery |
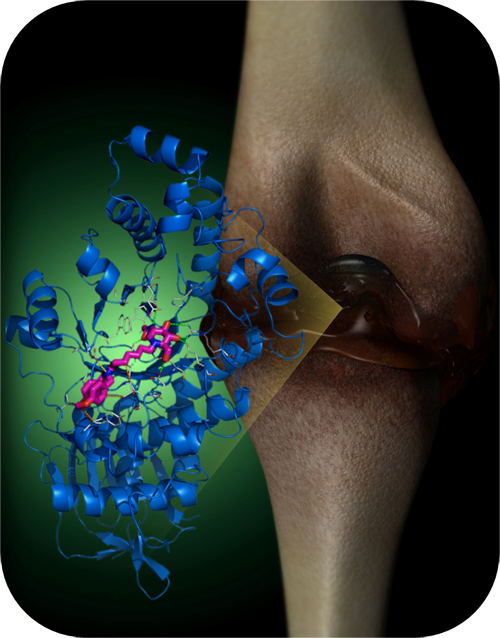
Human N-acetyl-£]-hexosaminidase (Hex) isozymes are considered to be important targets for drug discovery. They are directly linked to osteoarthritis because Hex is the predominant glycosidase released by chondrocytes to degrade glycosaminoglycan. Hex is also associated with lysosomal storage disorders. We report the discovery of GlcNAc-type iminocyclitiols as potent and selective Hex inhibitors, likely contributed by the gain of extra electrostatic and hydrophobic interactions. The most potent inhibitor had a Ki of 0.69 nM against human Hex B and was 2.5°—105 times more selective for Hex B than for a similar human enzyme O-GlcNAcase. These glycosidase inhibitors were shown to modulate intracellular levels of glycolipids, including ganglioside-GM2 and asialoganglioside-GM2.
|
 |
| Iminocyclitol |
£\-Fucosidase is associated with many disorders including inflammation, cancer, cystic fibrosis and fucosidosis. The enzyme is released by gastric epithelial cells only upon infection with Helicobacter pylori to affect the bacterial growth, adhesion, and pathogenicity. We report nine X-ray crystal structures in complex with iminocyclitols, which have Ki values spanning the micro- to picomolar ranges and up to 106-fold variation in potency. These inhibitors are C1- to C5- substituted fuconojirimacin derivatives. Some of them show time-dependent, slow-binding inhibition events that undergo progressive tightening of the enzyme-inhibitor complex from a low-nanomolar Ki value to a picomolar Ki*.
|
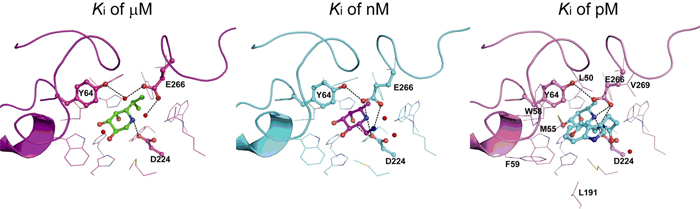 |
Among these X-ray structures, two loops were found to move inward toward the £\-fucosidase active site to produce a closed conformation of complexes with inhibitors with increasing Ki values from the micro- to nanomolar range. Although no further conformational changes in the two loops are observed for inhibitors with sub-nanomolar Ki values, the loops are additionally stabilized by hydrogen bonds and hydrophobic interactions. The increase in entropy and the flexibility of a glycon also play an important role. Our results clearly provide valuable insight in the design of potent enzyme inhibitors.
|
| Synthesis of Inhibitors |
|
°@ |
|
|
Browser & IE Recommendations: 1024*768 / IE 7.0°BFirefox 3.0
Add : 128 Sec. 2, Academia Road, Nankang,Taipei 115,Taiwan°@Tel : 886-2-27855696°@Fax : 886-2-27889759 |
|
Copyright © 2010 IBC. All rights reserved. |
|
 |
![]() |