| |
 |
| |
 |
|
Education and Experience:
|
|
|
|
2017-now : |
Research Fellow
Institute of Biological Chemistry, Academia Sinica |
|
|
2010-2017 : |
Associate Research Fellow
Institute of Biological Chemistry, Academia Sinica |
|
|
2003-2010 : |
Assistant Research Fellow
Institute of Biological Chemistry, Academia Sinica |
|
| 1999-2003 : |
Postdoctoral Fellow
Institute of Chemistry, Academia Sinica |
|
| 1993-1998 : |
Ph. D.
Department of Biochemistry, University of Cambridge, UK |
|
| 1992-1993 : |
Research Assistant
Institute of Biological Chemistry, Academia Sinica |
|
| 1989-1991 : |
M. Sc.
Institute of Biochemical Sciences, National Taiwan University |
|
| 1985-1989 : |
B. S.
Department of Agricultural Chemistry, National Taiwan University |
|
|
|
| |
Research interest:
My research interest is regarding protein folding and misfolding behaviors in order to answer how proteins can fold into its native structure and how certain proteins can misfold and cause disease. We focus on two diseases: prion disease and Alzheimer’s disease. We aim to tackle the biological problem from a chemist’s point of view. Currently we are studying the structure of prion fibrils, designing peptide inhibitor for inhibiting Aβ amyloid formation, and exploring the species barrier in prion disease transmission.
(1) Intranasal administration of a polyethylenimine-conjugated scavenger peptide reduces Aβ accumulation in a mouse model of
Alzheimer’s disease
Aβ aggregation in the brain plays a central and initiatory role in pathogenesis and/or progression of Alzheimer’s disease (AD). Inhibiting Aβ aggregation is a potential strategy in the prevention of AD. A scavenger peptide, V24P(10–40), designed to decrease amyloid β-protein (Aβ) accumulation in the brain, was conjugated to polyethylenimine (PEI) and tested as a preventive/therapeutic strategy for AD in this study. This PEI-conjugated V24P(10–40) peptide was delivered intranasally, as nasal drops, to four-month-old APP/PS1 double transgenic mice for four or eight months. Compared with control values, peptide treatment for four months significantly reduced the amount of GdnHCl-extracted Aβ40 and Aβ42 in the mice’s hippocampus and cortex. After treatment for eight months, amyloid load, as quantified by Pittsburgh compound B microPET imaging, was significantly decreased in the mice’s hippocampus, cortex, amygdala, and olfactory bulb. Our data suggest that this intranasally delivered scavenger peptide is effective in decreasing Aβ accumulation in the brain of AD transgenic mice. Nasal application of peptide drops is easy to use and could be further developed to prevent and treat Alzheimer’s disease (AD).
|
|
|
| |
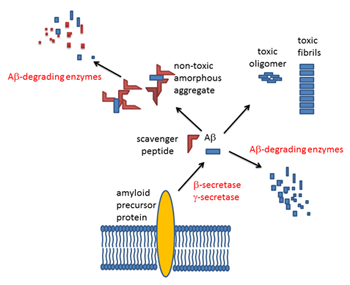
Proposed working mechanism of scavenger peptide on Aβ clearance
|
|
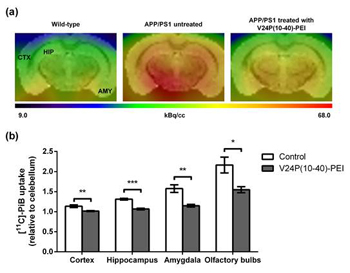
Effect of V24P(10-40)-PEI on inhibition of Aβ amyloid accumulation by PET scan
|
|
|
| |
(2) Polyhydroxycurcuminoids but not curcumin upregulate neprilysin and can be applied to the prevention of
Alzheimer’s disease
Neprilysin (NEP) is the most important Aβ-degrading enzyme. Its expression level decreases with age and inversely correlated with amyloid accumulation, suggesting its correlation with the late-onset of Alzheimer’s disease. Recently, many reports showed that upregulating NEP level is a promising strategy in the prevention and therapy of Alzheimer’s disease. Here, we used a sensitive fluorescence-based Aβ digestion assay to screen 25 curcumin analogs for their ability to upregulate NEP activity. To our surprise, four compounds, dihydroxylated curcumin, monohydroxylated demethoxycurcumin, and mono- and di-hydroxylated bisdemethoxycurcumin, increased NEP activity, while curcumin did not. The ability of these polyhydroxycurcuminoids to upregulate NEP was further confirmed by mRNA and protein expression levels in the cell and mouse models. Finally, feeding monohydroxylated demethoxycurcumin (also named demethylcurcumin) or dihydroxylated bisdemethoxycurcumin (also named bisdemethylcurcumin) to APPswe/PS1dE9 double transgenic mice upregulated NEP levels in the brain and reduced Aβ accumulation in the hippocampus and cortex. These polyhydroxycurcuminoids offer hope in the prevention of Alzheimer’s disease.
|
|
|
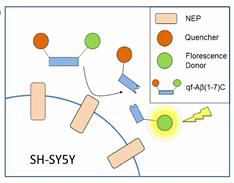
NEP activity assay
|
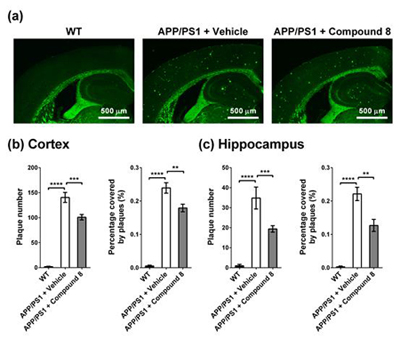
Effect of polyhydroxycurcuminoid on reducing amyloid plaque burden in the brain of the Alzheimer double transgenic mice.
|
|
|
| |
(3) Revealing structural changes of prion protein during conversion from α-helical monomer to β-oligomers by means of ESR
and nanochannel encapsulation
Under non-denaturing neutral pH conditions, full-length mouse recombinant prion protein lacking the only disulfide bridge can spontaneously convert from an α-helical-dominant conformer (α-state) to a β-sheet-rich conformer (β-state), which then associates into β-oligomers, and the kinetics of this spontaneous conversion depends on the properties of the buffer used. The molecular details of this structural conversion have not been reported due to the difficulty of exploring big protein aggregates. We introduced spin probes into different structural segments (three helices and the loop between strand 1 and helix 1), and employed a combined approach of ESR spectroscopy and protein encapsulation in nanochannels to reveal local structural changes during the α-to-β transition.
|
|
|
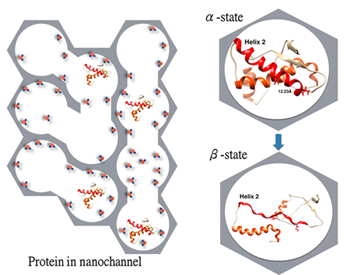
The the α-to-β transition of prion protein in nanochannels
|
| |
(4) Comparison of the anti-amyloidogenic effect of O-mannosylation, O-galactosylation, and O-GalNAc glycosylation
Our aim was to explore the effects of functional groups at carbon-2 (C2) of a sugar on the conformational properties of the peptide backbone. Three monosaccharides, mannose, galactose, and N-acetylgalactosamine (GalNAc), were added separately to the serine side-chain of a hamster prion peptide because it is a sensitive model for comparing the effect of protein modification on the conformational properties of the polypeptide chain. In buffer, this prion peptide goes through a gradual coil-to-β structural conversion and forms amyloid fibrils slowly during incubation. Our results showed that a sugar with an N-acetyl amino group in the equatorial configuration (GalNAc) or with a hydroxyl group in the axial configuration (mannose) on C2 had a greater inhibitory effect on the amyloidogenesis of the prion peptide than a sugar with the hydroxyl group in the equatorial configuration (galactose). We suggest that galactosylation has less effect than mannosylation or GalNAc glycosylation on promoting turn formation at the glycosylation site and on inhibition of amyloidogenesis. The anti-amyloidogenic property of mannose implies that protein mannosylation has an anti-aggregation function.
|
|
|
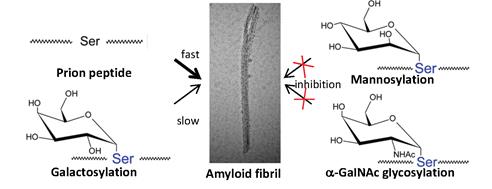
Effects of different glycosylations on the amyloidogenic property of prion peptide
|

|
|
Browser & IE Recommendations: 1024*768 / IE 7.0、Firefox 3.0
Add : 128 Sec. 2, Academia Road, Nankang,Taipei 115,Taiwan Tel : 886-2-27855696 Fax : 886-2-27889759 |
|
Copyright © 2009 IBC. All rights reserved. |
|
 |
![]() |
